Journal of Controlled Release (2022), corresponding-author, 11.467
Abstract
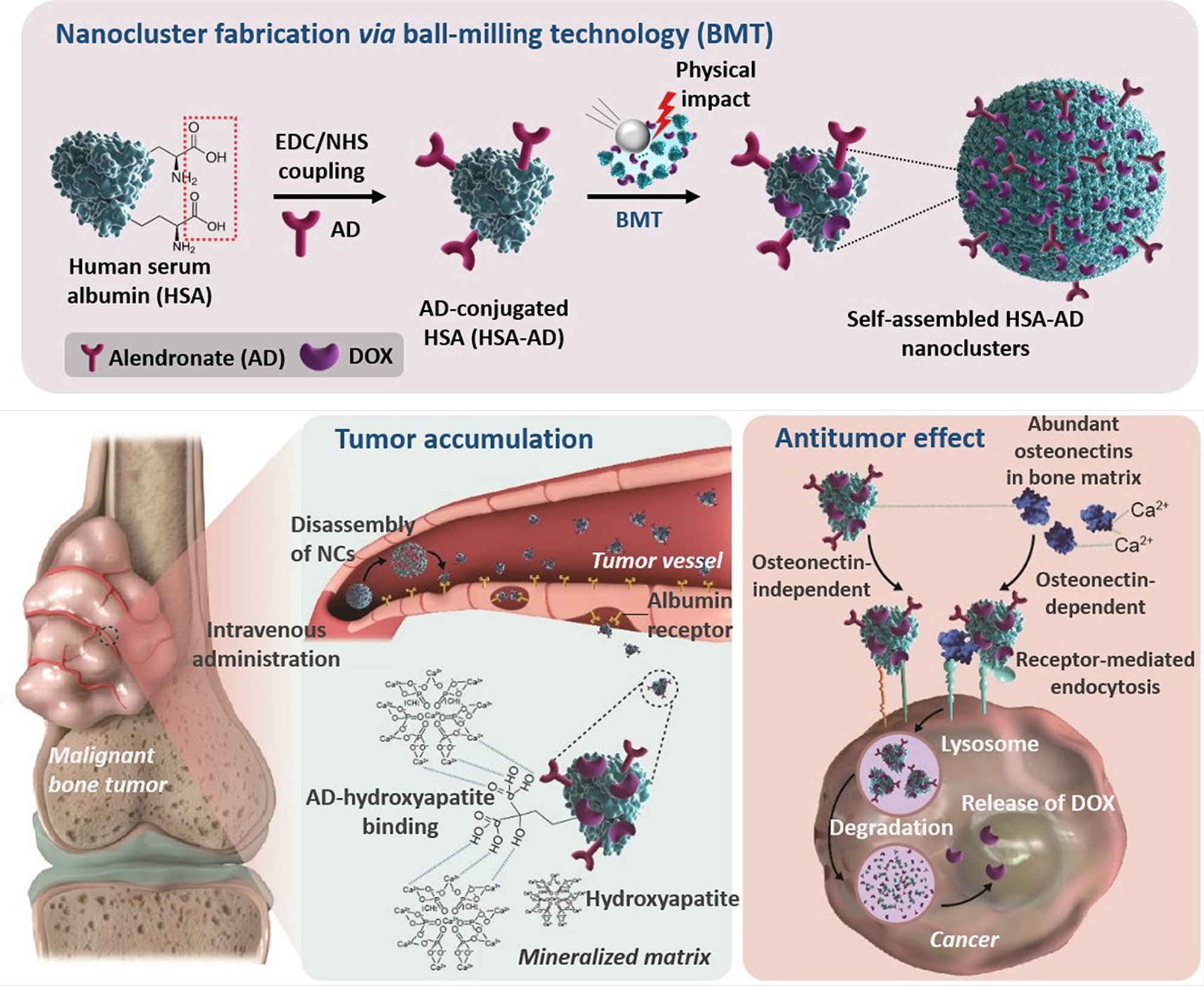
Hydroxyapatite-binding albumin nanoclusters (NCs) were developed for improving the anticancer agent accumulation in bone tumors. Human serum albumin (HSA) was decorated with alendronate
(AD), and doxorubicin (DOX)-loaded NCs (HSA-AD/DOX) were fabricated via the ball-milling technology, an innovative nano-fabrication method by which more than 90% of the secondary structures of albumin can be preserved. The targeting ability of NCs was confirmed using a novel in vitro bone cancer model, wherein hydroxyapatite and collagen, the major components of the bone matrix representing the highly mineralized bone tumor microenvironment, were co-cultured with HOS/MNNG, a human osteosarcoma cell line. The binding affinity of HSA-AD/DOX to hydroxyapatite was evaluated based on the DOX binding efficiency. HSA-AD/DOX showed a 5.04-fold higher affinity than HSA/DOX. The enhanced distribution of HSA-AD/DOX to bone tumors was verified using a newly developed mouse model bearing HOS/MNNG tumors with hydroxyapatite beads. HSA-AD/DOX led to a 52.0% increase in tumor accumulation compared to that of the unmodified HSA/DOX. This is mainly due to the hydroxyapatite-binding affinity of the AD moiety, which is supported by histological analyses performed on the dissected tumors. Furthermore, HSA-AD/DOX changed the protein expression patterns of the tumors, implying the enhanced apoptotic process. Overall, the targeting ability of HSA-AD/DOX are effectively translated into improved therapeutic efficacy in bone tumor-xenografted mice, suggesting that the developed NCs are a promising delivery system for bone tumor treatment.
Illustration of nanocluster fabrication using BMT and its mechanism of targeting the bone tumor microenvironment
.png&blockId=484d951f-4a74-4ba5-ac0c-97a71142f0d7)